
Reviewed by Emily Henderson, B.Sc.
Researchers at the Scheie Eye Institute in the Perelman School of Medicine at the University of Pennsylvania released initial data from a new clinical trial. The data suggests a new gene therapy for one of the most common forms of congenital blindness is safe and improved patients’ vision.
The therapy delivers working copies of GUCY2D to the eyes of patients who have severe vision impairments caused by mutations in the gene.
What is gene therapy?
Gene therapy is an experimental technique that uses genes to treat or prevent disease. In the future, this technique may allow doctors to treat disorders by inserting a gene into a patient’s cells instead of using drugs or surgery. Researchers are testing several approaches to gene therapy, including:
- Replacing a mutated gene that causes disease with a healthy copy of the gene.
- Inactivating, or “knocking out,” a mutated gene that is functioning improperly.
- Introducing a new gene into the body to help fight a disease.
Although gene therapy is a promising treatment option for a number of diseases (including inherited disorders, some types of cancer, and certain viral infections), the technique remains risky and is still under study to make sure that it will be safe and effective.
For the time being, gene therapy will most likely be limited to patients with rare diseases or specific mutations. The Food and Drug Administration (FDA) has only approved a limited number of gene therapies for use in the U.S. So, it’ll be a while before these treatments are widespread.
Researchers are mostly testing gene therapy for diseases that have no other cures, such as congenital blindness.
What is the GUCY2D gene?
The GUCY2D gene is one of about 25 different human genes whose mutations cause problems in the retina, leading to severe vision impairment from birth or early childhood. This family of inherited retinal disorders, collectively known as Leber congenital amaurosis (LCA), accounts for a considerable portion of blindness in children worldwide.
The GUCY2D gene provides instructions for making a protein that plays an essential role in normal vision. This protein is found in the retina—the specialized tissue at the back of the eye that detects light and color. Within the retina, the GUCY2D protein is located in light-detecting cells called photoreceptors. The retina contains two types of photoreceptor cells: neurons known as rods and cones. Rods are needed for vision in low light, while cones are needed for vision in bright light, including color vision.
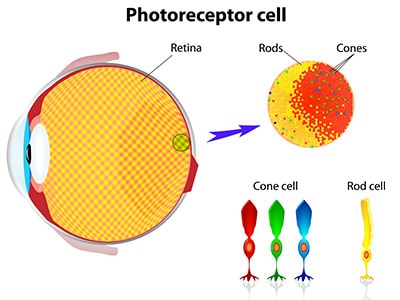
The GUCY2D protein is involved in a process called phototransduction. When light enters the eye, it stimulates specialized pigments in photoreceptor cells. This stimulation produces an electrical signal which the optic nerve carries to the brain. The brain interprets this signal as vision. Once photoreceptors have been stimulated by light, they must return to their resting (or “dark”) state before they can be stimulated again. The GUCY2D protein is involved in a chemical reaction that helps return photoreceptors to their dark state after light exposure.
Normal copies of GUCY2D encode an enzyme in the key pathway used to convert light into electrochemical signals. A lack of this enzyme blocks the pathway’s recovery, preventing the reset needed for further signaling. Therefore, the signal from rod and cone cells becomes very weak—which causes severe vision loss.
Even in adults who have lived with this condition for decades, many light-sensing retinal cells may remain alive and intact despite their dysfunction. Thus, adding functional copies of GUCY2D via gene therapy could get those cells working again and restore some vision.
About the study
In 2019, Jacobson and co-investigator Artur V. Cideciyan, Ph.D., a research professor of Ophthalmology in the Perelman School of Medicine, began the first clinical trial of a GUCY2D gene therapy. The therapy is a single injection of a harmless viral vector that carries the gene. The solution is injected beneath the retina—initially in just one eye per patient.
They have been following each patient for two years after treatment. In the new 2021 report, they described their findings after nine months in the first three patients treated.
What happened to the three study participants?
- The first patient experienced a substantial increase in light sensitivity in rod cells in the treated eye. Rod cells are more light-sensitive than cone cells and are chiefly responsible for low-light or “night vision.” This patient also showed improved pupil responses to light.
- The second patient showed a smaller but sustained increase in light sensitivity in rod cells. The improvement started about two months after the gene therapy.
- The third patient showed no improvement in rod cell sensitivity. However, they did show significantly improved visual acuity over the nine-month follow-up period. The researchers tied this improvement to better function in the patient’s cone cells, the predominant cells for daylight and color vision.
“These initial results from the first-ever trial of a GUCY2D gene therapy are very encouraging and will inform our ongoing and future trials of this therapy,” said Cideciyan. There were no serious adverse side effects. Any side effects that occurred in the patients’ retinas resolved.
The gene therapy dose used in these first three patients was the lowest possible dose. The researchers plan to use higher doses going forward. So, they are hoping to see continued safety and greater efficacy in later-enrolled patients who will receive higher doses.
The ongoing clinical trial is registered at clinicaltrials.gov as trial NCT03920007.
Why Choose Assil Gaur Eye Institute for your eye care?
The doctors at AGEI offer world-class eye care and vision correction, specializing in emergency eye care, LASIK vision correction, treating cataracts, glaucoma, and a wide variety of cornea and retinal conditions, to name a few. At AGEI, you will experience state-of-the-art medical facilities that bring together revolutionary technologies with experienced ophthalmologists. Our goal is to help you achieve your personal best vision.
Please call 866-945-2745 or visit us here to make an appointment online if you are experiencing any concerning symptoms to determine the best time to schedule an exam.
At AGEI we take our patients’ safety seriously. That’s why our facility’s Covid-19 patient safety procedures exceed all CDC recommendations.
We are conveniently located throughout Southern California and the Los Angeles area. Our ophthalmologists are available at locations in or near Santa Monica and Beverly Hills. We are conveniently located near West Los Angeles, Culver City, West Hollywood, Downtown Los Angeles, Marina del Rey, Pacific Palisades, Malibu, Manhattan Beach, Sherman Oaks, and Encino.
Article Source:
Jacobson, S.G., et al. (2021) Safety and Improved Efficacy Signals following Gene Therapy in Childhood Blindness Caused by GUCY2D Mutations. iScience. doi.org/10.1016/j.isci.2021.102409.














