- Home
- Resource Library
- Retina Resources
- Valeda Light Delivery System
Valeda Light Delivery System for Dry Age-Related Macular Degeneration (AMD)
There is New Hope for Patients with Dry Macular Degeneration!
Dry age-related macular degeneration (AMD) is an eye condition that impairs central vision by damaging the macula. The macula is the central part of the retina responsible for sharp, straight-ahead vision and the ability to see fine details. This type of vision is used when looking at faces, reading text, or driving.
Dry AMD is a leading cause of vision loss among older adults over the age of 55, and nearly 11 million Americans are living with Dry AMD today. It is projected to double by 2050. To date, treatment options for dry AMD have been limited.
Dry AMD progresses slowly and is classified into stages:
Early AMD
Small yellow deposits called drusen begin to form under the retina. There is typically no vision change.
Intermediate AMD
As drusen accumulate, they can interfere with the function of the retinal cells and lead to thinning and atrophy of macular tissue. Larger drusen and/or pigment changes appear, and some vision changes may be noticeable.
Advanced AMD
Retinal cells deteriorate, leading to geographic atrophy; notable vision loss is possible at this stage.
How does the Valeda Light Delivery System work for treating Dry age-related macular degeneration (AMD)?
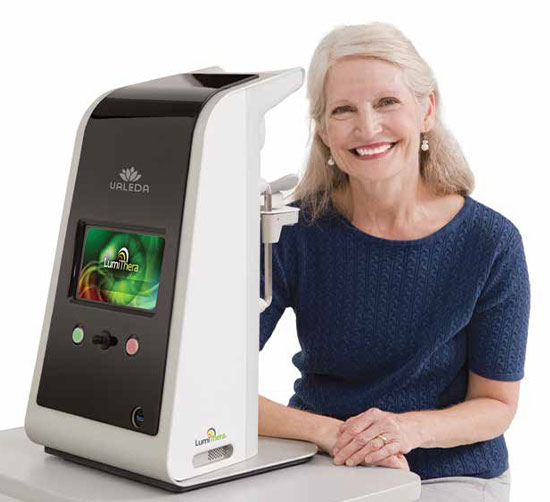
Valeda from LumiThera is a revolutionary first and only FDA-approved new treatment device for dry AMD that uses gentle, targeted light to help prevent progression and improve vision in dry AMD.
This medical device utilizes photobiomodulation (PBM), specifically targeting the red, amber, and near-infrared wavelengths of light. PBM therapy produces mitochondrial stimulation in retinal cells. This helps your retinal cells increase their energy production and stay healthier for longer.
Although there is no cure for dry AMD, early treatment remains crucial. Valeda therapy has been shown to slow the progression of the disease and can even enhance your vision, improving your quality of life.
Who is suitable for the VALEDA treatment?
VALEDA is indicated for use in the treatment of dry age-related macular degeneration (AMD) patients, especially those experiencing mild-moderate vision impairment. Individuals receiving this treatment should have already been diagnosed with dry AMD by a retinal specialist. The stage of dry AMD may impact the benefits of the treatment.
Valeda is intended for individuals with early to intermediate dry AMD, typically those with mild to moderate vision loss (e.g., 20/32 to 20/70), and who have drusen (yellow deposits) or non-central geographic atrophy.
Valeda is not intended for patients with Wet AMD (those receiving eye injections).
Who is NOT a good candidate for the VALEDA treatment?
- Patients who have any known light exposure reactions or a history of light-sensitive central nervous system disorders, such as epilepsy or migraines, are not able to receive this treatment.
- Patients using any photosensitizing medications (e.g., topical creams, certain antibiotics, or injectables) should consult their physician first and generally must wait 30 days or more before receiving this treatment.
Our retinal specialist, Dr Svetlana Pilyugina, will help you determine if you are a good candidate for this treatment during your consultation.
What is involved in the VALEDA treatment?
What is the VALEDA treatment like?
- The Valeda treatment is non-invasive, quick, and comfortable, lasting approximately 15 minutes per session.
- It does not require eye dilation or injections and has no recovery downtime.
- A typical treatment course has three treatments per week for a total of nine sessions over 3–5 weeks
- Maintenance treatments may be needed in 4-6 months
Clinical trials have shown improvements in visual function after treatment three times a week for 3-4 weeks. This is the recommended treatment protocol for patients with Dry AMD.
In a recent clinical study, patients received no more than three treatments per week and no more than one treatment within a 24-hour period. If you miss a treatment, it is recommended that you make up the session within the 3–4 week period of the treatment series.
Do I need to do anything special to prepare for VALEDA treatments?
Not really. If you wear glasses or contact lenses, you will be asked to remove them before your treatment. Your eyes will not be dilated.
Are there any side effects of the VALEDA treatment?
Photobiomodulation (PBM) is a low-level, light-based therapy that enhances cell function. PBM treatment has been used in many different diseases and disorders for decades and has a positive safety profile.
In clinical trials in dry AMD patients, the use of light therapy has also been shown to be safe. VALEDA is designed to be eye safe. There have been no treatment-related side effects noted in previous studies with the VALEDA. Over 800,000 treatments have been administered worldwide to date with no reported side effects.
What can I expect during and after the VALEDA treatment?
It is well known that looking at bright light can produce an afterimage. Another name for this is photobleaching, where the cells that “see” that specific color become fatigued.
For example, if you look at a red light, those colors may fade after treatment, and you will see more of the opposite color (i.e., green). This phenomenon is typically observed immediately following treatment and normally resolves within a couple of minutes.
You can resume normal activities immediately after this non-invasive treatment.
Clinical Effectiveness & Safety
Valeda clinical trials (the LIGHTSITE III study) showed very promising results: patients experienced improvements in vision (gaining letters on an eye chart) and slower disease progression compared to those who received a sham treatment (the doctor goes through the motions but does not actually perform the treatment).
The treatment has shown a strong safety profile, with no serious side effects reported during the trials. FDA authorization came in November 2024, marking it as a significant milestone: the first non-invasive, light-based therapy approved for dry AMD.
Assil Guar Eye Institute is the best treatment center for VALEDA and macular degeneration in Los Angeles.
Have you or someone in your life been wondering whether there’s anything new to offer patients with dry macular degeneration? Or have you felt that you had no real treatment option for your Dry AMD? Now, there is new hope.
Valeda, a light therapy approach that’s safe, non-invasive, and shown to slow vision loss (and even improve vision in some cases for people with early or intermediate dry AMD), is now available at AGEI.
Although it’s not a cure, it offers hope, slows disease progression, and increases the likelihood of preserving vision for a longer period. Please schedule a consultation with our Stanford-trained Retinal Specialist, ophthalmologist Dr. Svetlana Pilyugina, to see if you might be a candidate.
Please call (866) 945-2745 or request your appointment online to schedule a consultation with our ophthalmology retinal specialist, so we can determine if Valeda is right for you.
VALEDA FAQs
Can I have VAEDA treatments if I have cataracts?
Yes. Patients can receive the VALEDA treatment regardless of whether they have cataracts.
How long might the benefits of VELEDA treatments last?
This depends on factors such as the stage of your disease at the start of treatment, individual disease progression, your adherence to the treatment protocol, and whether you come for the recommended maintenance treatment sessions.
Is VALEDA covered by insurance or Medicare?
As this treatment has relatively recent FDA approval, there is a possibility that your Medicare or private insurance may decline coverage. In such cases, you may need to pay out of pocket or explore financing options













