- Home
- Resource Library
- Glaucoma Resources
- OMNI® 360 Glaucoma Treatment System
OMNI® 360 Glaucoma Treatment System
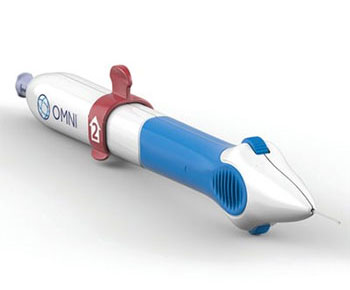
What is the OMNI® Glaucoma Treatment System?
The FDA Approved OMNI® Glaucoma Treatment System is the next-generation Minimally Invasive glaucoma surgery (MIGS).
Developed by Sight Sciences, the OMNI 360 is the only device that combines two well-established minimally invasive glaucoma surgery (MIGS procedures) into one using a single device and a single clear corneal incision.
It reduces intraocular pressure in adult patients with open-angle glaucoma. It can be used for mild, moderate, and severe primary and secondary open-angle glaucoma.
- Let's quickly review glaucoma
- Not all Glaucoma surgery is the same
- How is the OMNI® Glaucoma System different than other glaucoma surgeries?
- Post-op care for the OMNI® Glaucoma Treatment System is easy
- OMNI Surgical System FAQs
- Assil Gaur Eye Institute’s glaucoma specialist voted Los Angeles’ Best Ophthalmologists
Let's quickly review glaucoma
Glaucoma is an irreversible condition where the optic nerve, which helps your brain form a picture for you to see, becomes damaged by a buildup of pressure in the eye. It can lead to a loss of vision and, ultimately, blindness. So, it's very important to treat early.
Not all Glaucoma surgery is the same
Minimally invasive glaucoma surgery, better known as MIGS, is considered to be safer and less invasive than traditional glaucoma surgery. "Minimally invasive" means that tiny incisions and/or microscopic equipment are used. These procedures are generally safer compared to traditionally invasive surgery.
As a MIGS procedure, the OMNI® Surgical System helps restore the natural flow of fluid from the eye to lower pressure and reduce damage to the optic nerve without a large incision.
How is the OMNI® Glaucoma System different than other glaucoma surgeries?
The OMNI 360 Glaucoma System is different in several important ways, which include:
Titratable Therapy Facilitating up to 360° of Canal Treatment
- Treats all three points of resistance, the trabecular mesh-work, Schlemm’s Canal, and the distal collector channels, to reduce IOP
- Viscodilates up to 360° of Schlemm’s canal
- Unroofs up to 360° of the trabecular mesh-work
Efficient Minimally Invasive Canal Surgery
- Ab-interno approach spares the conjunctiva and sclera of incisions
- Implant free and sutureless
- Stand-alone procedure or combined with cataract surgery in a single OR session
The doctors at Assil Gaur Eye Institute were among the first doctors in the nation to perform this procedure post clinical trials. Because it is minimally invasive, it has a quick recovery. Most patients see a significant response because of the two mechanisms of action.
Post-op care for the OMNI® Glaucoma Treatment System is easy
Besides your regular post-op follow-up visits with your doctor, patients are given various drops to use for up to 4 weeks and to sleep with their head raised at a 30-degree angle for two to three weeks. If combined with cataract surgery, some additional cataract procedure drops will be given.
If you are one of Assil Gaur Eye Institute’s glaucoma patients and are having cataract surgery and you have glaucoma, talk to your doctors about MIGS and the Omni Surgical System for glaucoma. If you are a patient of ours, you can be assured that we will talk to you about it.
If you are having trouble controlling your eye pressure or would prefer to have a laser or a minimally invasive glaucoma procedure over medications or major surgery, talk to our doctors about MIGS and the Omni Surgical System for glaucoma.
View the OMNI 360 Surgical System brochure here.
OMNI Surgical System FAQs
Q: What is the OMNI Surgical System?
The OMNI Surgical System is a safe and minimally invasive surgical procedure that helps reduce intraocular pressure (within the eye) in adult, primary open-angle glaucoma patients by opening up and restoring flow through the eye’s natural drainage pathway. This procedure can be performed at the same time as cataract surgery or on its own as a standalone glaucoma procedure.
The procedure is implant-free, allowing the eye’s natural drainage system to safely restore pressure. In several clinical trials, OMNI has proven consistent in lowering eye pressure in patients by greater than 20%1, and, in some cases, OMNI even eliminated the need for drops after surgery2.
Q: How does the OMNI Surgical System reduce eye pressure?
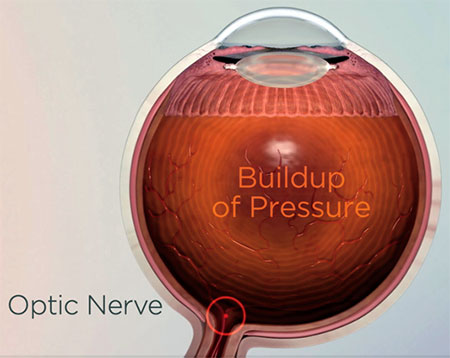
Glaucoma is the result of damage to the optic nerve. The damage is usually due to an increase in eye pressure caused by a blockage in the eye’s natural drainage canals. The blockage is like a clog in the kitchen sink. When fluid isn’t able to drain properly, it builds.
The OMNI Surgical System helps an ophthalmologist “unclog the sink”, or relieve pressure buildup in the eye by removing unnecessary blockages. OMNI works to help restore the eye’s natural drainage system by removing unnecessary blockages across several pressure points in the eye.
Q: What should I expect during and after the procedure?
The OMNI Surgical System is a minimally invasive outpatient procedure that can be performed at the same time as cataract surgery. Unlike many other minimally invasive glaucoma procedures, OMNI can be performed on its own, even if cataract surgery has already been done.
This procedure can be completed in a matter of minutes under local anesthesia, which numbs the eye to ensure comfort. Although every patient is different, recovery time is usually quick, similar to recovery from cataract surgery. As an outpatient procedure, you’ll go home the same day and have a follow-up visit, usually the day after surgery.
Q: What are the possible side effects of the OMNI procedure?
Minimally invasive glaucoma surgery, better known as MIGS, may be safer and less invasive than traditional glaucoma surgery. "Minimally invasive" means that microscopic equipment is used with these procedures, generally making them safer compared to traditionally invasive surgery. The most common side effects of the OMNI procedure may include temporary elevation in pressure as the eye begins to normalize and bleeding in the eye.
Q: What are alternative treatment options for glaucoma?
Treatment options depend on the type of glaucoma, severity, and personal preference. Treatment options range from oral and topical medications (like eye drops) and laser
procedures to minimally invasive glaucoma surgery (MIGS) and more invasive surgeries, like trabeculectomies and some implants.
Many patients struggle to take their drops every day and/or manage the costs associated with those medications. By naturally restoring the eye’s drainage system with OMNI, the dependence on drops has the potential to be reduced or even ended. If you continue to experience elevated eye pressure or struggle to use drops daily, a minimally invasive option like the OMNI Surgical System may be right for you.
Q: How quickly does the procedure start to work?
The OMNI Surgical System restores the eye’s natural drainage system, allowing many patients to experience a reduction in eye pressure soon after the procedure. In some cases, the pressure in the eye takes a bit to normalize after surgery. It is not uncommon to remain on drops for a period of time after surgery. Your doctor will work with you to determine if medications can be reduced or potentially stopped long-term.
Every patient is different, so your doctor will schedule routine check-ins following the surgery to test your eye pressure and evaluate your progress after the procedure.
Q: Am I a good candidate for the OMNI procedure?
The OMNI Surgical System is indicated to reduce ocular pressure in adult patients with primary open-angle glaucoma. Individual results may vary. In order really to know if you are truly a good candidate, talk to your doctor about whether the OMNI Surgical System is right for you.
Assil Gaur Eye Institute’s glaucoma specialist voted Los Angeles’ Best Ophthalmologists
Named one of Los Angeles’ Top Ophthalmologists for 2020 and 2021, Dr. Avneet K. Sodhi Gaur is the head of our Glaucoma Service. She is a Board Certified, Fellowship trained Cataract, LASIK (refractive surgery), and Glaucoma Specialist. Her surgical technique training and experience developed at nationally renowned ophthalmology institutions, including Tufts Medical Center/New England Eye Center and Ophthalmic Consultants of Boston-Mass Eye and Ear Infirmary.
Dr. Sodhi Gaur has extensive experience in all types of glaucoma surgical procedures, such as trabeculectomy, glaucoma drainage implants, primary open-angle glaucoma, and, of course, the Omni 360 Treatment. She also performs state-of-the-art minimally invasive glaucoma procedures, including endocycloph.
Setting up an appointment with us is easy. Simply click here to request an appointment online or call 866-945-2745. CDC recommendations during this coronavirus pandemic. Masks are required in our institutes at all times.
We are conveniently located for eye care patients throughout Southern California and the Los Angeles area at locations in or near Beverly Hills, Santa Monica, West Los Angeles, West Hollywood, Culver City, Hollywood, Venice, Marina del Rey, Malibu, Manhattan Beach, and Downtown Los Angeles.
Sources
1. Brown RH, Tsegaw S, Dhamdhere K, Lynch MG. Viscodilation of Schlemm canal and trabeculotomy combined with cataract surgery for reducing intraocular pressure in open-angle glaucoma. J Cataract Refract Surg. 2020 Apr;46:644-645.
2. Vold SD, Williamson BK, Hirsch L, Aminlari AE, Cho AS, Nelson C, Dickerson JE Jr. Canaloplasty and Trabeculotomy with the OMNI System in Pseudophakic Patients with Open-Angle Glaucoma: The ROMEO Study. Ophthalmol Glaucoma. 2021;4:173-181. doi: 10.1016/j.ogla.2020.10.001.
-
Dr. Gaur's training and work experience at renowned ophthalmic institutions, including Tufts Medical Center and Boston-Mass Eye and Ear Infirmary, have given her extensive experience in state-of-the-art medical, laser and surgical management of glaucoma and cataracts. It is no exaggeration to report that she has performed thousands of sucessful cataract, glaucoma and LASIK surgeries.















